possibilities
Test procedures
Our comprehensive facilities allow the testing of various devices, transducers and equipment from all areas of hearing acoustics. Our testing activities are focussed on
- type testing for approval in product group 13 (hearing assistive devices) in the list of aids published by the National Association of Statutory Health Insurance Funds (GKV-Spitzenverband) in Germany,
- testing of hearing aids according to IEC 60118-0,
- testing of hearing aids for use in noisy environments according to the testing principle DGUV 312-002.
We are also open for all kinds of individual requests.
The German Institute of Hearing Aids (Deutsches Hörgeräte Institut GmbH) in Lübeck is a test house accredited by the Deutschen Akkreditierungsstelle (DAkkS) according to DIN EN ISO/IEC 17025 and recognized by the Zentralstelle der Länder für Gesundheitsschutz bei Arzneimitteln und Medizinprodukten (ZLG) according to the Regulation (EU) 2017/745 or Medical Device Regulation (MDR). The institute has laboratories and acoustic test rooms for the homologation of hearing aids and for research. For some tests, the certificates can also be issued in English. An example is the test according to IEC 60118-0, which includes not only the signet of the DAkkS and ZLG but also the ILAC signet.
Possibilities
Hearing Aids
For type testing and testing according to IEC 60118-0, hearing aids are measured in an anechoic chamber using a 2cm³ coupler according to IEC 60318-5. Depending on the task, the measurement can also be conducted with an ear simulator according to IEC 60318-4, a high-frequency coupler according to IEC 60318-8, or special couplers. An example of a special coupler is a custom-developed coupler for measuring active occlusion cancellation. Further, measurements can be conducted on an artificial head according to IEC 60318-7, in a diffuse field chamber or in a test box.
Possibilities
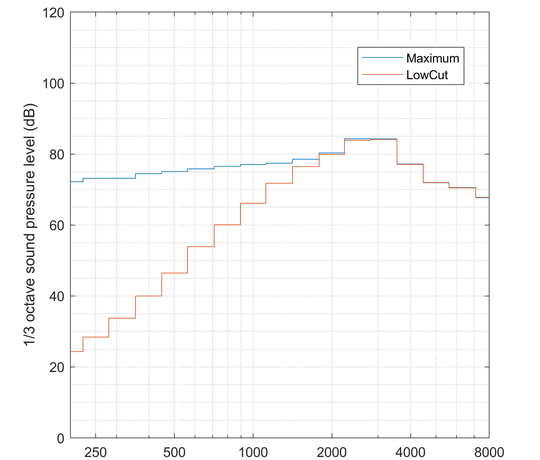
Tinnitus aids
For tinnitus aids, the output signal is measured in third octave bands following IEC 60118-0. Further evaluations usually comprise the spectrum, possible adjustments and the maximum output level.
Possibilities
Bone conduction hearing aids
For testing bone conduction hearing aids, the artificial mastoid according to IEC 60318-6 and the skull simulator SKS-10 are available in our institute. This allows the regular testing of all established types of bone conduction devices, like hearing glasses, headbands, transducers with adhesive coupling or bone-anchored hearing aids.

Possibilities
Headphones & hearables
For supraaural and circumaural headphones, the ear simulator according to IEC 60318-1 and the 6mc³ coupler according to IEC 60318-3 are available. Further, earphones or hearables can be tested in the ear simulator (IEC 60318-4), 2cm³ coupler (IEC 60318-5) or high-frequency coupler (IEC 60318-8). All ear-worn devices may also be tested on an artificial head (IEC 60318-7). We also have implemented measurement procedures to evaluate active features in earphones and hearables, such as hear-through, Active Noise Cancallation (ANC) and Active Occlusion Cancellation (AOC).










